Return to CHC Home
Page
Nattokinase
to
Reverse Atherosclerosis
Nattokinase (NK), at a dose of 300 mg (6000 Fibrin Units or 3/32
tsp.) two times a day will:
A. Decrease carotid arterial plaque area (by up to 36%) within one year.
B. Regress carotid artery intima-media-thickness (1.33 to 1.04 mm in one study).
C. Protect against lower extremity venous clot (DVT) formation.
D. Lowers LDL (18%) and triglycerides (16%) and raises HDL (16%).
E. Improve clinical outcome following ischemic stroke.
F. Lowers BP slightly (»
10%).
G. Provide benefit in chronic venous insufficiency.
Benefits in animal models that should apply to humans:
A. Dissolves (experimental) arterial and venous clots (not in minutes but within
hours).
B. Decreases oxidative/inflammatory stress, with less cell loss and enhanced
recovery, following stroke (and presumably with any other occlusive arterial
event).
C. Decreases risk of clot formation and mitigates organ damage in infection.
D. Protection against Alzheimer’s.
E. Protection against proliferative retinopathy.
F. Decreases damage and enhances recovery with gamma irradiation or toxin
exposure.
The clinical effects occur on the basis of NK’s physiologic
effects:
A. Fibrinolytic and Anti-Thrombotic– NK dissolves fibrin clot and fibrinogen
(fibrin precursor).
B. Blood viscosity and RBC aggregation decreases (your blood turns from catsup
to wine-like).
C. Improves endothelial function and attenuates endothelial thickening after
arterial injury.
D. Antioxidant (protection against lipid peroxidation) and tissue oxidative
stress.
E. Anti-platelet effect (limits thromboxane A2 release).
F. Neuroprotective effect (degrades amyloid fibrils and promotes neurogenesis).
G. Degrades the Covid spike protein.
H. Blunts angiotensin II generation.
I. Degrades HMG Co-A Reductase.
J. Attenuates inflammatory and oxidative stress: Blocks the TLR4®NF-kβ
& MAPK and NLRP3®Il-1β, Il-6, and TNFα cascades while enhancing the
restorative NRF-2®HO-1 cascade.
K. Modifies apoptosis (cell suicide) and protects against necroptosis (cell
death).
Dosing:
A. 50 mg (1000 Fibrin Units) protects against DVT but does not affect
atherosclerosis.
B. 200 mg (4000 FUs) three times a day or 300 mg (6000 FUs) twice a day will.
C. Take ≥ 30” before protein containing meals (the NK could get used up
digesting the protein).
D. If weight < 125 lbs. take 150-200 mg twice a day.
Risk and Concerns:
A. Two case reports of abnormal bleeding due to NK.
B. Hold NK when you need to be a good clotter (trauma, nose bleed, 2 days
pre-surgery).
C. Aspirin synergizes with NK and does not increase bleeding risk.
D. We have no studies documenting safety (wrt bleeding risk) of NK taken along
with Warfarin, oral anti-coagulants, or anti-platelet drugs (all of which are
associated with bleeding risk).
E. In animals, doses up to 100 mg/kg/day (7,000 mg/day in a 70 kg adult) without
toxicity.
F. Doses up to 10 mg/kg (700 mg/day in adult) without any toxicity in human
trials.
Logistics:
A. Obtain NK from purebulk.com (powder form) or Allergy Research Group
(capsules).
B. NK will digest protein so take at least 30” or 2 hours post-meals.
C. NK can be taken with amino acids, including glycine and NAC within the GlyNAC
protocol, and non-protein supplements or pharmaceuticals.
D. Aspirin 81 mg and Vit K2 synergize with NK wrt plaque regression.
E. Effects begin
»
4-6 hours and last
»
8-12 (thus this is not a once-a-day
agent).
F. While NK is obtained from soybean fermented with B. subtilis, there is no soy
protein in NK.
G. Cost should be
»
50 cents/day (from purebulk).
What does Dr. Roberts take?
Nattokinase 300 mg (3/32 tsp) + N-Acetylcysteine (4000 mg) + Glycine 4000 mg (1
tsp of each) twice a day in juice.
Key study: Observation of the efficacy of nattokinase in patients with carotid
atherosclerosis and hyperlipidemia. Ren, H, et. al. Chinese Medical Journal
2017. doi: 10.3760/cma.j.issn.0376-2491.2017.26.005.
Ren and colleagues randomized 82 subjects to NK 3,000 FU (150 mg)
twice a day or Simvastatin 20 mg once a day. At one year, Simvastatin had a
stronger LDL-lowering effect
(- 27 % vs. - 13% with NK), while NK decreased carotid plaque area from 0.25 to
0.16 cm2
(- 36% vs. - 12% with statin) and reversed CIMT from 1.13 to 1.01 mm (-
11%) vs from 1.12 to 1.07 (-5%) with statin. Of note, treatment-induced LDL
reduction did not correlate with the degree of plaque or IMT reduction.
Side-effects did not occur.
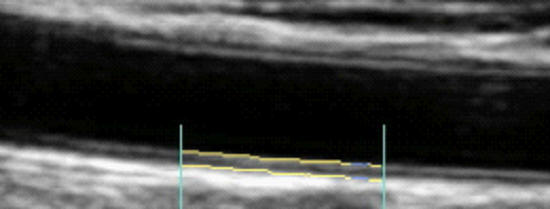
Effect of Nattokinase on Carotid Intima-Media Thickness and Plaque Expression


Carotid Artery Intima-Media Thickness (brackets, above), the combined thickness of the endothelial and muscular layers of the Common Carotid Artery, reflects your current propensity to form plaque and risk of future atherosclerotic events (heat attack, stroke, etc.). IMT increases with age, on average at 0.01 mm/year, and IMT rate of change relates to risk factor control efficacy. Slow progression or stabilization of IMT reflects good risk factor control. Conversely, rapid IMT progression indicates that we have more work to do. All phenomena shown to increased CV risk will increase IMT while effective therapeutic efforts will attenuate IMT (discussed in detail elsewhere on this website).
While IMT is systemic, atherosclerotic plaque is focal, and above we see mild plaque at the level of the carotid bulb. Change in arterial physiology (risk factor control) determines IMT, which in turn determines rate of plaque progression and overall atherosclerotic risk.
Three studies have looked at the effect of Nattokinase (at different doses) on IMT and Common Carotid Artery plaque progression. While carotid plaque is not coronary plaque, directional change in carotid plaque will correlate with plaque progression/regression elsewhere within one's vasculature.
♥ Ren and colleagues randomized 82 middle-aged, asymptomatic,
hyperlipidemic (none on lipid-lowering therapy) subjects to a one year program
of:
·
Simvastatin 20 mg once a day, or
·
Nattokinase 150 mg
(3,000 FUs) twice a day
Lipids, Carotid Artery Intima-Media Thickness (CIMT), and common carotid plaque area were measured at baseline and at one year.
No important side-effects were encountered. Specifically, abnormal bleeding was not recorded in either group.
What did Dr. Ren discover?
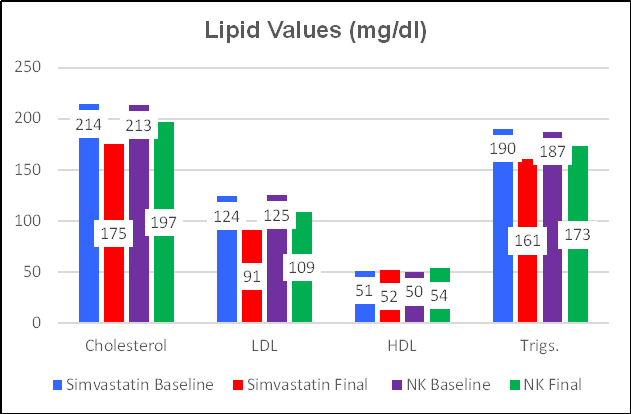
Simvastatin (a "statin" drug) and
Nattokinase both inhibit HMG Co-A Reductase, the rate limiting step in
cholesterol synthesis. Simvastatin provides a stronger effect, lowering
total cholesterol by 18%, from 214 to 175 mg/dl, vs. an 8% drop with
Nattokinase. Similar findings were observed with respect to LDL and triglyceride
reduction. Nattokinase, but not Simvastatin, produced a significant rise
in (protective ) HDL. So far, the lipid-lowering edge goes to Simvastatin, but we will keep in
mind that only 10% of plaque is compromised of cholesterol.
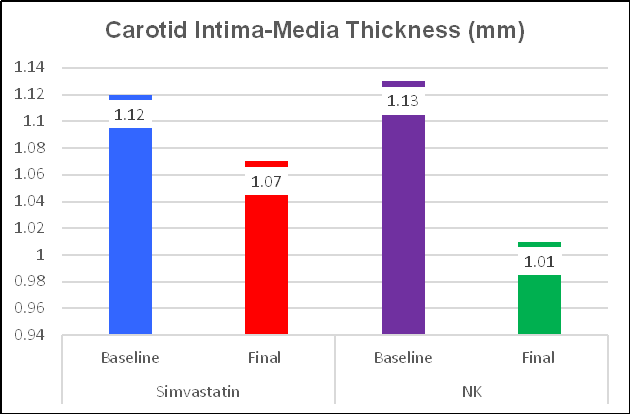
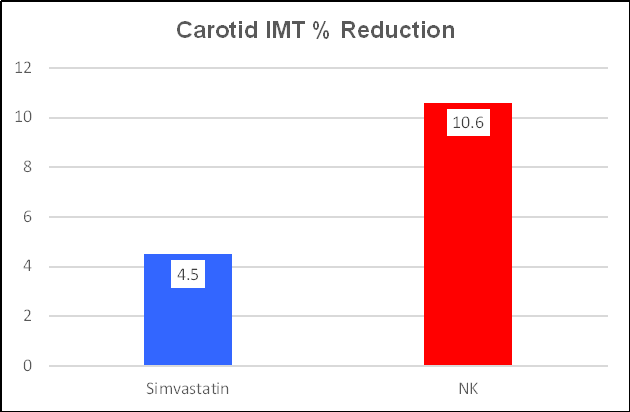
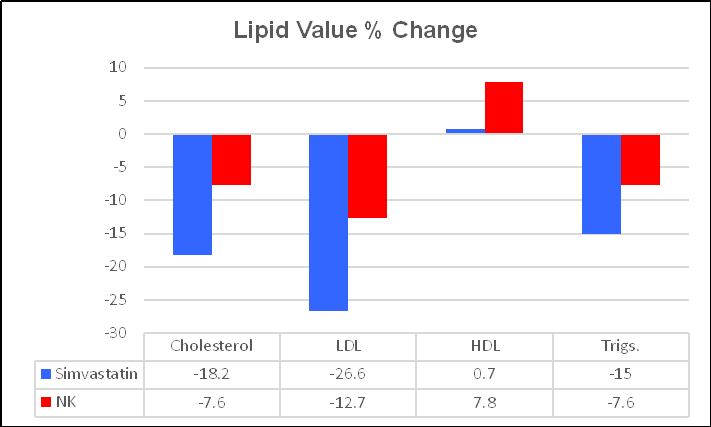
Simvastatin was effective, reducing IMT by 4.5%, from 1.12 to
1.07, or 0.05 mm/year. NK was more effective, decreasing IMT by 10.6%, from 1.13
to 1.01, or 0.12 mm/year. These one-year changes are greater than what we record
in the office, and may related to methodological differences (this study was
carried out in China), but the directional changes are obvious. NK was twice as
effective as the drug Simvastatin at regressing IMT! As change in IMT
should translate to change in plaque expression, what do you think Ren observed
with respect to plaque area?
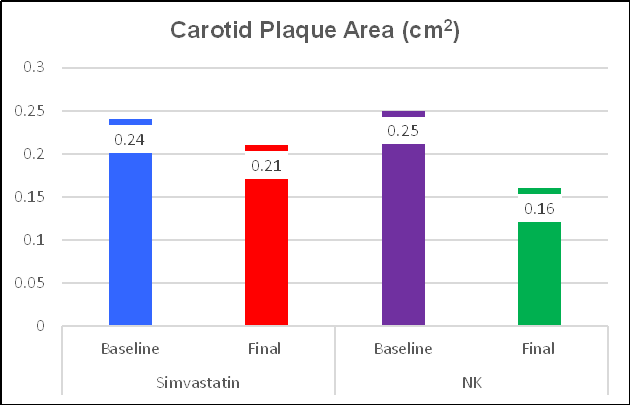
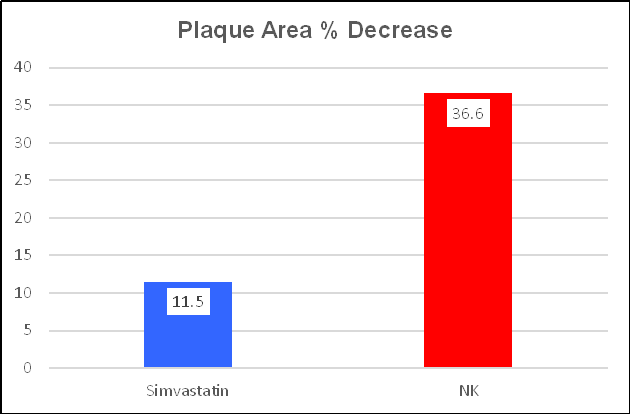
Simvastatin decreased plaque expression, over one year, by 11.5%, a good thing. NK was 3 & 1/2 times more effective, decreasing plaque expression by 36.6%. Ren noted that change in lipids, in relation to either therapy, did not correlate with change in IMT or plaque expression (cholesterol is just one part of this disease). IMT change relates to risk factor control, to recent vascular biology, and LDL is just one factor. Simvastatin lowers LDL and attenuates oxidative/inflammatory stress. NK provides weaker LDL control, but stronger oxidative/inflammatory stress effects. NK has a multitude of beneficial effects not provided by statin drugs, as outlined above and discussed in more detail below.
Thus if you had to choose one approach, statin therapy vs. nattokinase, the logical choice would be nattokinase, Of course, we do not need to make this choice. We can do both (and we can use drug or non-drug LDL reducing agents), along with a multitude of other anti-atherosclerotic approaches available to us within our framework of Integrative Cardiology.
♥ Chen and colleagues reviewed the charts of 1,062 proactive clinic patients, all of whom were treated with Nattokinase (NK) over a one-year time frame. All had undergone lipid and carotid artery assessments at baseline and at one year. All were hyperlipidemic (but not on lipid-lowering treatments), 2/3rds carried a diagnosis of sub-clinical atherosclerosis (like nearly all of you), 181 were taking Vitamin K2 (at a standard dose of 180 mcg/day), 96 were taking 100 mg/day of aspirin, 259 were smokers, 82 were obese, and 706 exercised on a regular basis.
A minority of Chen's subjects were taking what we now realize to be
an insufficient NK dose (180 mg = 3,600 FU/day), which provided insignificant
lipid lowering and a greatly attenuated IMT effect. The majority of Chen's
subjects took what we now realize to be the effective dose (180 mg three times a
day). This provided 10,800 FUs per day, a little more than Ren's subjects (who
received 6,000 FUs).). Can you predict what Chen found?
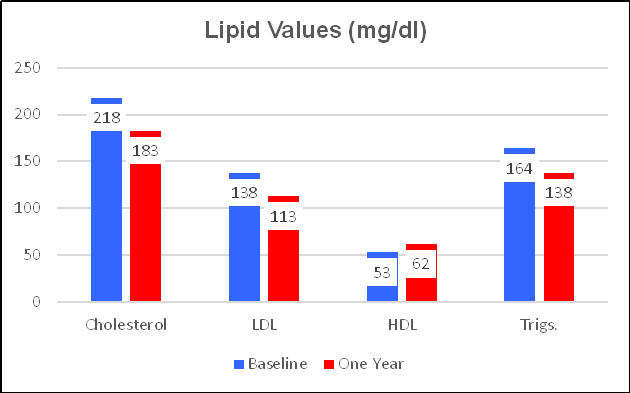
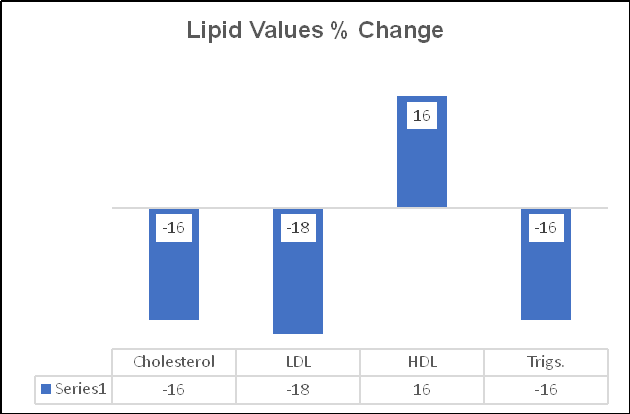
Ren found that 300 mg (6,000 FUs) of NK lowered LDL by 13%. Chen's subjects took 540 mg (10,800 FUs), which lowered LDL by 18%, and increased HDL by 16% (vs. 8% on the lower, Ren dose). This should not surprise us. Escalating doses of BP and lipid-lowering interventions provide escalating effects; the same with Nattokinase.

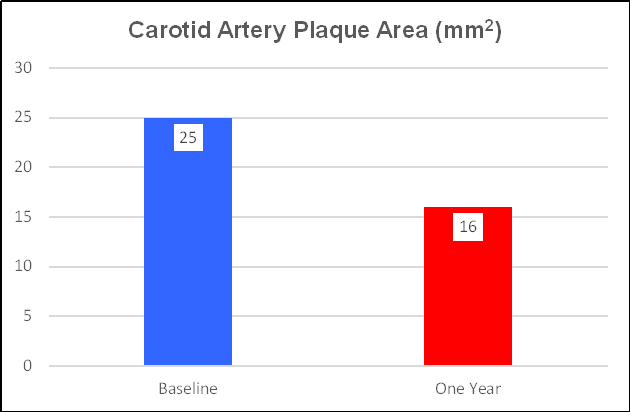
CIMT decreased by 25%, from 1.22 to 1.04 mm, a one-year progression rate of - 0.18 mm/year. Plaque area regressed by 36% (as did Ren's cohort).
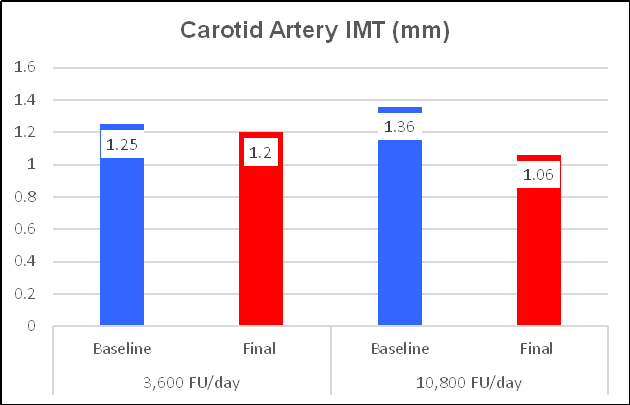
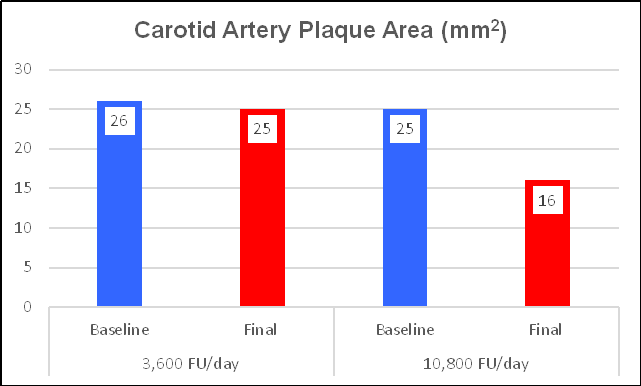
Getting back to the Nattokinase dose-response relationship, Chen's subjects who took the lower NK dose did experience a benefit, with a minimal reversal of IMT and plaque expression, but clearly less then with NK at 540 mg/day.
Exercisers and those who took aspirin and/or Vitamin K2 experienced a stronger NK effect (as exercise, aspirin, and K2 work at different levels of atherosclerosis control). Smokers and obese patients experienced an above-average treatment effect (they had more upside potential). All of this makes sense.
♥ Hodis and associates spent a lot of money demonstrating that an insufficient dose of NK had little effect. 265 middle-aged subjects received 100 mg (2,000 FUs) of NK or placebo, and underwent CIMT testing every six months over three years. Nothing happened. There were no side-effects, and there was no NK effect on CIMT, BP, or lipid parameters.
Why did Hodis use such a low dose? We learned years ago that low-dose (50 to 100 mg/day) NK protects against lower-extremity venous thrombosis (DVT), and that NK works here by dissolving venous thromboses (blood clots). So, if NK dissolves blood clots, why wouldn't NK increase our risk of abnormal bleeding? It turns out that NK does not increase bleeding risk, but given our (prior) concern that it might, and our knowledge that 50-100 mg is safe, we limited NK dosing to this level (often termed the "European Dose"). In Japan and China, where intake of NK is common place, and bleeding is not observed (outside of two case reports), Ren, Chen, and other researchers felt comfortable with the higher doses, and so now can we. Thus, if you are troubled by atherosclerosis, clinical or sub-clinical, and are not taking prescription blood-thinners, it makes sense to take NK at a dose of 200 mg three times a day or (as I do) 300 mg twice a day (once a day dosing is inappropriate as the effect begins to attenuate at 8-12 hours). We decrease our recommended doses by 1/3rd if you weight less than 120 lbs. Taking NK alone with oral anti-coagulants and anti-platelet agents has not been studied with respect to safety. NK probably would not increase bleeding risk, but I do not know this, and thus we would add NK to these agents only in specific situations (rapid disease progression or breakthrough clot formation while on drug therapy).
Observation of the efficacy of nattokinase in
patients with carotid atherosclerosis and hyperlipidemia. Ren, H, et. al.
Chinese Medical Journal 2017. doi: 10.3760/cma.j.issn.0376-2491.2017.26.005.
Effective management of atherosclerosis progress and hyperlipidemia with
nattokinase: A clinical study with 1,062 participants. Chen, H. et. al. Front
Cardiovasc Med. 2022 Aug 22:9:964977. doi: 10.3389/fcvm.2022.964977.
Nattokinase atherothrombotic prevention study: A randomized controlled trial.
Hodis, H. et. al. Clin Hemorheol Microcirc. 2012;78(4):339-353. doi:
10.3233/CH-211147.
Effect of Nattokinase on Stroke Recovery and Neurogenesis
Transient Ischemic Attack (TIA) refers to transient and Cerebral Vascular Accident (CVA or Stroke) to permanent loss of function on the basis of a vascular event. Symptoms are sudden in onset, and may involve loss of vision in the eye served by a blocked carotid artery (termed amarosis fujax), or loss of function over the opposite side of the body (termed hemiplegia).
Hemorrhagic stroke, devastating but fortunately uncommon, relates to bleeding within the brain. Lacunar, or "small vessel" stroke, relates to the combined effects of atherosclerosis, aging, and hypertension. Embolic strokes occurs when a blood clot (thrombus) mobilizes from the heart (primarily in the setting of atrial fibrillation). Ischemic stroke occurs when blood flow is interrupted by micro-clot formation within an atherosclerotically narrowed vessel.
Treatment of acute stroke relates to its mechanism. In the ER, a brain CT will be carried out. If hemorrhage is excluded, you may receive rTPA (recombinant, or laboratory generated, Tissue Plasminogen Activator). TPA is released by vascular cells, in response to local clot initiation, and converts latent Plasminogen into Plasmin, our natural clot dissolving enzyme. Like TPA, rTPA activates Plasmin, rapidly and throughout the vasculature. The clot compromising brain perfusion will dissolve (termed clot lysis), blood flow is restored, and stroke symptoms will resolve. The down-side here is that appropriate clot formation (at your IV site or within an internal organ, such as a gastric ulcer) will falter, such that bleeding may occur. The sooner rTPA is administered, the better. rTPA given within four hours of stroke onset should lead to brain recovery; beyond 12 hours little benefit will be achieved. In between, rTPA is a risk to benefit decision, balancing preservation of brain function vs. risk of bleeding.
Like rTPA, Nattokinase (NK) will activate Plasminogen, within 4-6 hours if taken orally. In animal models of stroke or experimental clot formation, IV Nattokinase works as well as does rTPA, but with less bleeding potential. Given the lag time with oral NK, if stroke symptoms, you would not rely on oral NK. Rather you would call 911 and get to the hospital as soon as possible. If you were out in the wilderness, hours away from medical care, with reasonable certainty that your stroke symptoms were ischemic and not hemorrhagic, then taking in 600 mg of NK would make sense, expecting clot lysis in 4-6 hours.
In this section we are not going to discuss NK as a non-clot-lysis approach to acute stroke (as IV NK is not available). We are going to discuss NK as an approach to promote stroke recovery, and (in animal studies) to limit stroke-related brain damage, on the basis of the multiple non-thrombolytic beneficial effects of NK on our physiology.
♥ Pham and colleagues randomized
61, 30-70 year-old, Vietnamese patients with completed (outside the window for
rTPA) hemiplegic (one-sided weakness) stroke to a 60-day program of:
·
Standard care (physical therapy,
electroaccupuncture, and daily IV
Naatrapyl, an inotropic GABA derivative, not utilized in the US,
+ placebo, or
·
Standard care + Nattokinase
600 FUs (30 mg) twice a day
Standard stroke severity/recovery ratings (Rankin, Barthel, and Orgogozo) were obtained at baseline and and again at 30 and 60 days.
The standard care and standard care + NK groups were evenly matched for age, stroke severity, and risk factor profile
HR and BP fell slightly in both groups, no more so with NK. Cholesterol decreased from 213 to 184 mg/dl with standard therapy and a little more, from 212 to 179 mg/dl, with NK, not an earth shattering difference. Side-effects were not identified. Specifically, abnormal bleeding did not occur. No changes in standard lab studies were seen.
An element of stroke recovery (restoration of lost function) occurred in both groups, but to a greater extent in the NK patients.
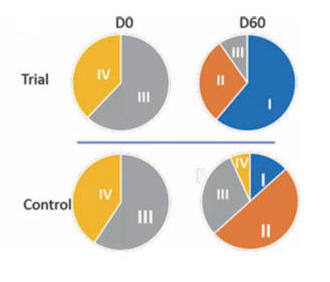
The Rankin scale measures functional deficit on a 1-IV scale, with IV reflecting serve impairment and I only mild neurologic loss.
The chart below shows that a far greater percentage of the NK stoke subjects improved into Rankin I.
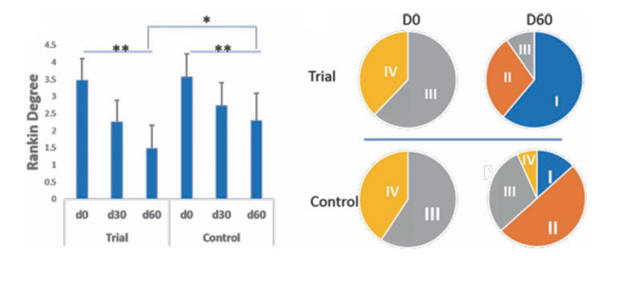
Similar benefits were observed within the Barthel and Orgogozo scales.
Pham has demonstrated that negligible-cost, risk-free, Nattokinase improves functional recovery following stroke - Great! But why is this occurring?
In this setting NK is not working by dissolving the blood clot that caused the stroke. NK may certainly dissolve residual clot, but Pham did not treat his stroke patients in the emergency room, but rather after the stroke had been completed, after all the clot-related damage that could be done had occurred.
NK worked in Dr. Pham's patients on the basis of its' myriad tissue protective and neurogenic (stimulates generation of new brain cells) effects, as documented in the animal stroke-intervention studies abstracted below.
Nattokinase: A Promising Alternative in Prevention and Treatment of Cardiovascular Diseases. Chen, H. et. A. Biomarkers Insights Vol. 13:1-8.
Post-stroke neurological recovery, and the avoidance of post-stroke depression and dementia (not uncommon occurrences over the first year post-stroke) is dependent on neurogenesis, the generation of new, functional brain cells, from in situ brain stem cells. These mono-potent, tissue-specific (they can make brain but not non-brain cells) arose from the pluri-potent (can give rise to any cell) VSEL (Very Small Embryonic-Like) pool at day seven post-conception (please see our VSEL-Activation section as to how we can stimulate this process in adulthood). Brain stem cells localize within specific brain locations, the peri-ventricular (adjacent to the CSF containing ventricles) and within the mid-line hippocampal region. Hippocampal neurogenesis is involved not just in stroke recovery, but in mitigating dysfunction in other brain disease states (Alzheimer's, Parkinson's, Huntington's Disease, and aging itself). Inflammation and oxidative stress compromise neurogenesis, while neurogenesis is enhanced by BDNF (Brain-Derived Neurotrophic Factor). Specific phenomena (Lithium, Lions' Mane mushroom, and exercise) will increase expression of BDNF. In the study abstracted below, Wu and colleagues demonstrate that Nattokinase increases expression of BDNF, with consequent neural protection and enhanced neurogenesis, thus improving post-stroke outcome.
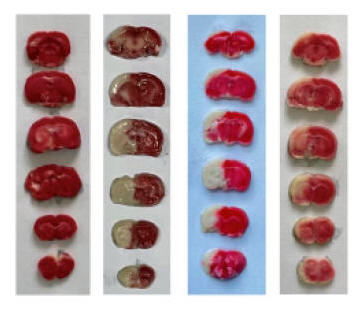
♥ Dr. Wu and his colleagues fed healthy, six-week old rats standard chow or chow supplemented with NK 2500 FU/kg or 5000 FU/kg (which would equate to 350,000 FU in a 70 kg adult human) over one week (and noted no bleeding or any other adverse effect). Acute stroke was then carried out by ligating closed the right internal carotid artery over 4, 8, or 24 hours. Some of the animals were sacrificed, to allow quantification of brain tissue loss and brain biochemistry, while others were observed over the ensuing 24 days, to see if NK pre-treatment affected stroke recovery and overall neurological status.
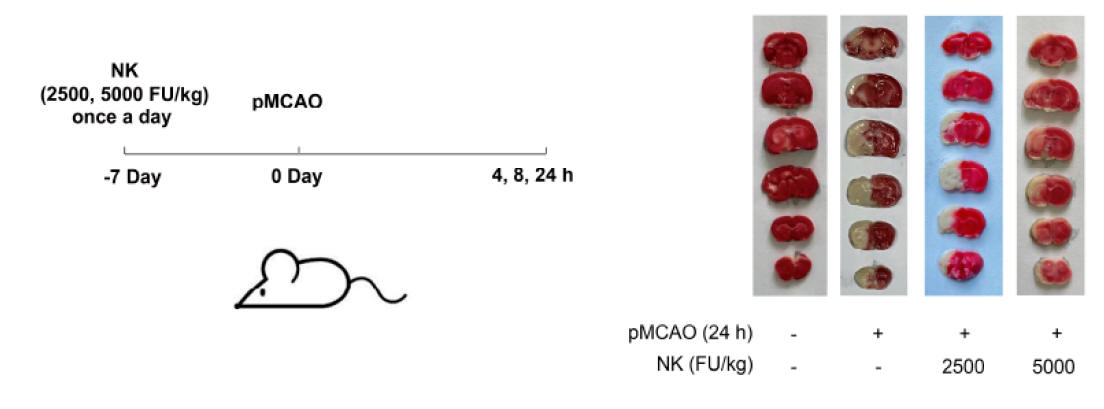
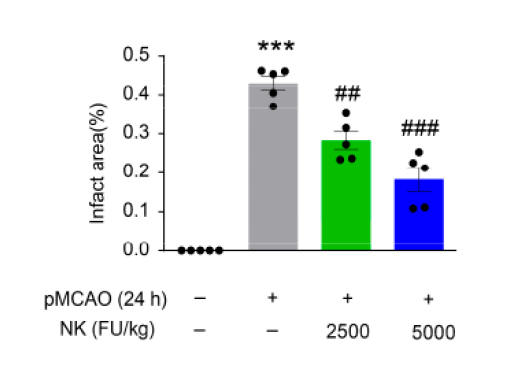
Above left recapitulates the experimental design, The middle
graphic contains brain scans, taken 24 hours post experimental ischemic stroke,
with the darkish tissue reflecting normal brain and the whitish tissue dead
brain, with quantification on the right. Similar findings were noted on the 4
and 8 hours scans. Pre-treatment with NK thus protected against brain loss
due to sudden carotid artery closure.
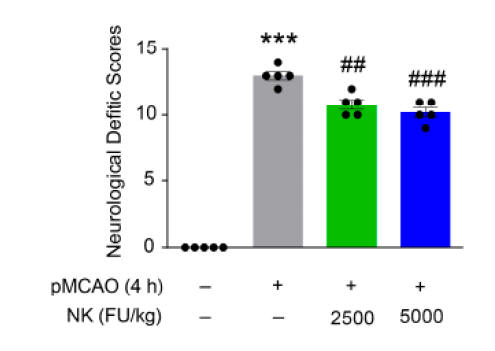
The above graphic depicts 24-hour post-stroke neurological dysfunction. With less brain loss we see less dysfunction. Similar benefits were noted at four weeks.
Different stains can be applied to the brain tissue, to quantify different cell types or physiologic phenomena, and with this methodology Wu demonstrated, four weeks out from experimental stroke, enhanced neurogenesis (the generation of new brain cells) within the hippocampus, enhanced myelination (the material that insulates nerve cells), and enhanced angiogenesis (new blood vessel formation) in relation to NK supplementation.
How did NK increase cerebral BDNF? NK does not cross the blood-brain barrier, but it increases brain BDNF, protects against cell loss, and promotes neurogenesis. We got interested in NK three decades ago, with the understanding that NK will lyse (split up) fibrin and fibrinogen, providing a clot preventing and clot-lysing effect. We are learning now that NK will lyse a number of deleterious proteins, such as amyloid fibrils, TLR4 and NLRP1, inflammation amplification platforms, and the Covid spike protein. Wu demonstrated that NK will cleave the large, inactive, circulating protein FNDC5, into Irisin, a small protein that will cross the blood-brain barrier, where it acts to increase BDNF expression, and other pathways involved in NK-stimulated stroke recovery and neurogenesis. NK promotes resilience/recovery in a number of health challenges, and Wu and colleagues hypothesize that NK-induced Irisin generation serves as a mediator.
Nattokinase Promotes Post-Stroke Neurogenesis and Cognition Recovery via Increasing Circulating Irisin. Wu, H. et. al. J. Agric. Food Chem. 2023, 71, 11418-11428.
While Wu demonstrated a benefit of NK supplementation pre-stroke, Ji and colleagues demonstrated NK-brain protection, with post-stroke (post brain ischemic-reperfusion injury) with NK supplementation, and depicted several mechanisms of NK-benefit..
♥ Dr. Ji and colleagues assigned healthy, 300 gm., adult male Wistar rats into one of five groups:
A. Sham surgery.
B. MCAO (unilateral ligation of the middle-cerebral artery over two hours)
followed by reperfusion for 24 hours.
C. MCAO over two hours followed by 72 hour reperfusion combined with NK at 9.4
or 4.7 mg/day, begun 4, 24, or 48 hours post-reperfusion.
D. MCAO over two hours followed by 72 hour reperfusion combined with
Lumbrokinase (LK) at 0.4 KU/day
Occlusion followed by reperfusion generates an "oxidative storm", characterized by high level generation of superoxide and other reactive oxygen species, which in turn accelerate cellular damage. The graphic below depicts brain tissue chemistry at 24 hours, with NK given 4 hours post-stroke, and demonstrates an obvious anti-oxidant effect. Lumbrokinase is presented elsewhere on this website, and like NK, has been shown to promote stroke recovery and to provide anti-atherosclerotic effects. These agents should be, in general, functionally interchangeable, but LK is far more expensive, and is currently less extensively studied, so we have shifted from LK to NK use.
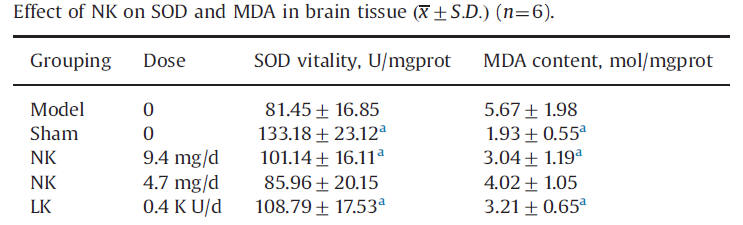
Super Oxide Dismutase (SOD) breaks down Super Oxide, a powerful and potentially damaging free radical, into less toxic, and easier to neutralize, hydrogen peroxide. SOD levels fall with brain ischemia-reperfusion (IR) injury (this happens in the heart during heart attack). Malondialdehyde (MDA), a marker of cell membrane lipid oxidation, consequently rises. NK, in a dose-dependent fashion, and LK, both blunt this IR -induced fall in SOD and rise in MDA, blunting tissue damage.
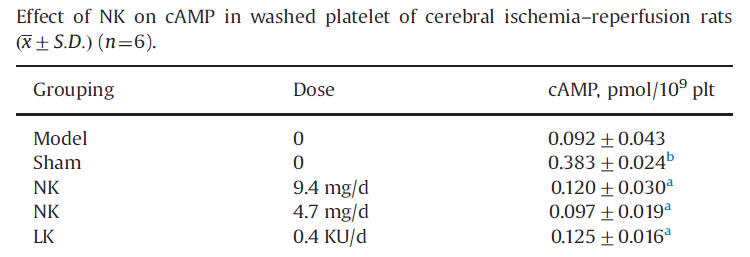
Platelet activation, initiating the clotting cascade, is mediated by a rise in cAMP, an intra-platelet messenger. cAMP rise with IR stroke, and this rise is blunted by NK and LK - thus less tendency for post-stroke clot formation within the cerebral microvasculature.
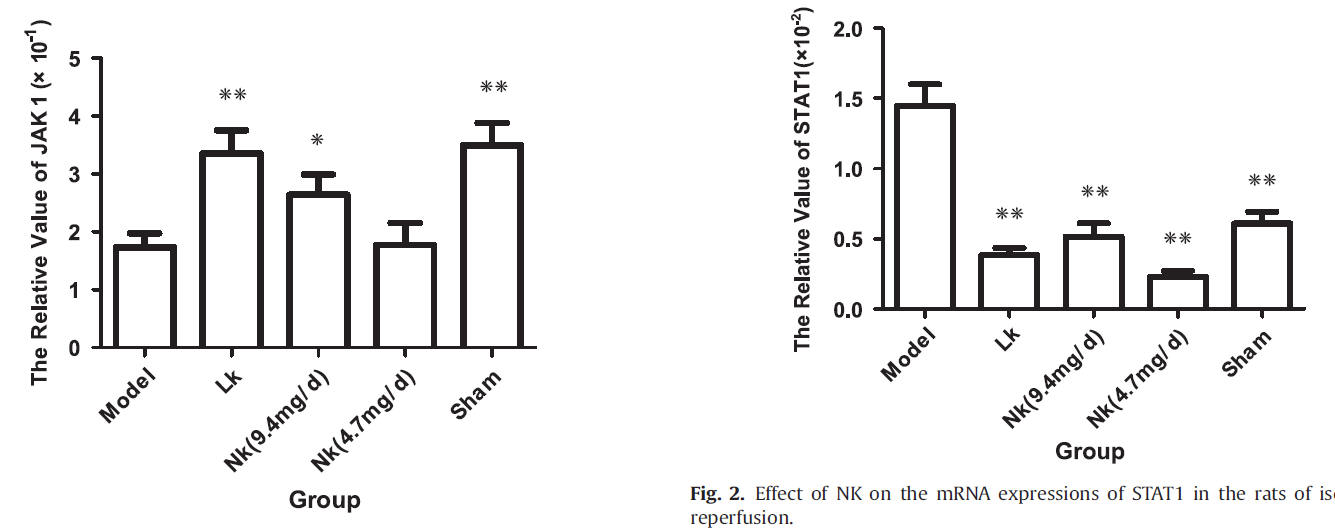
The enzyme JAK1 is expressed in brain development and maturation stages of mammals. JAK1 promotes cell resilience, cell survival, and blunts cell apoptosis (cell demise in response to stressors). JAK1 falls with experimental stroke, and this fall is ameliorated by LK and by NK in dose-dependent fashion. STAT, on the other hand, promotes apoptosis (cell suicide). STAT rises with IR, and this is ameliorated by NK and LK.
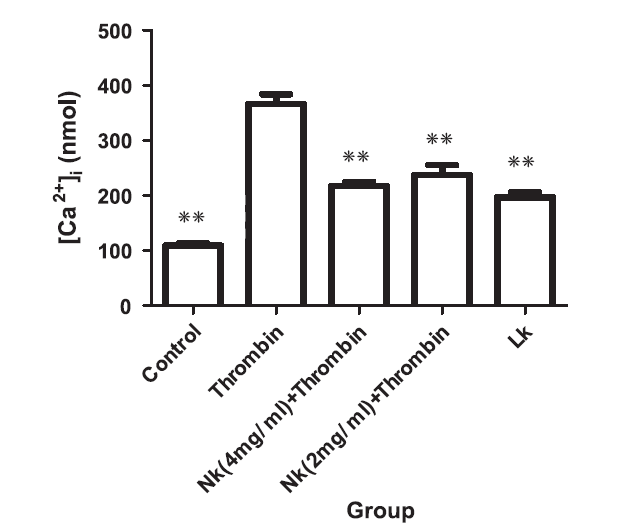
Thrombin initiates the clotting cascade, in part by activating platelets, by increasing intra-platelet calcium concentration. Ji treated human platelets, pre-loaded with NK or LK, with thrombin, and demonstrated partial protection against thrombin-induced intra-platelet calcium spike (thus demonstrating an anti-platelet effect of NK and LK).
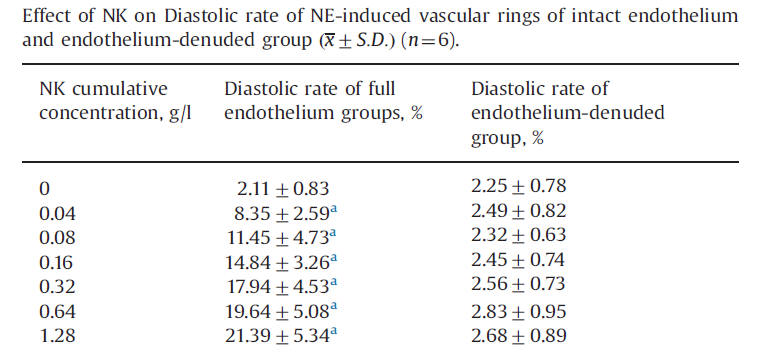
Endothelial function, the "risk factor of risk factors" is presented in detail elsewhere on this website. Intact endothelial function protects against atherosclerosis and clot formation while endothelial dysfunction mediates vascular injury. Ji demonstrated endothelial protection by NK, in relation to the ability of NK, again in a dose-related fashion, to promote relaxation of vascular smooth muscle in the presence of norepinephrine-induced vasoconstriction. Thus NK is endothelial cell protective (this has been shown in other animal models a well).
Mechanisms of Nattokinase in protection of cerebral ischemia. Ji, H, et. al. European Journal of Pharmacology 745 (2014) 144-151.

In stroke, heart attack, or any
other form of tissue injury, there is a deleterious cross-talk between between
the inflammation, coagulation, and oxidative stress cascades. These
pathways are designed to protect us against the threats experienced by primitive
main (infection and trauma), and conspire against us in "new to evolution"
health challenges that face us today (vascular disease, toxicity, and diabesity)
Dr. Yang and colleagues looked a the effect of NK on these interactions.
♥ Dr. Yang and colleagues assigned healthy, 18-weel old, Sprague Dawley rats into one of five groups:
A. Sham surgery.
B. tMCAO (unilateral ligation of the middle-cerebral artery over two hours)
followed by reperfusion.
C. tMCAO in animals supplemented over seven days pre-tMCAO with NK at 5,000,
10,000, or 20,000 FU/day (the latter dose would equate to 7 million FU is an
adult).
Stroke size, brain chemistry, and functional recovery were subsequently assessed.
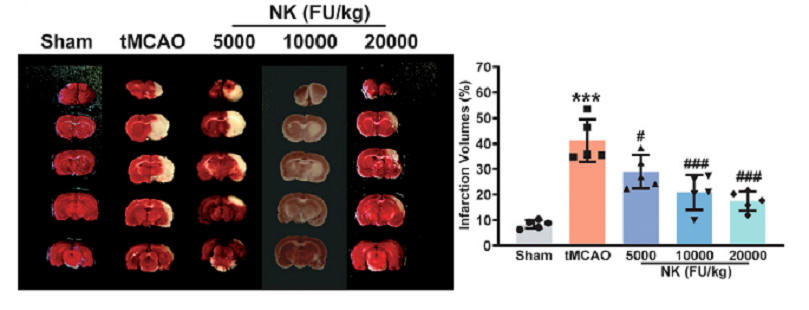
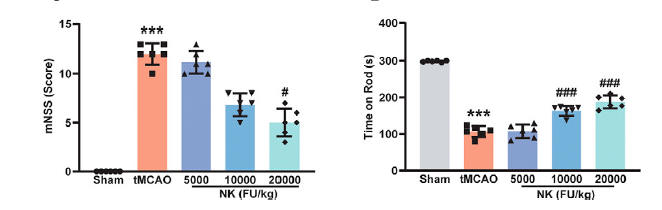
Less brain tissue is lost with NK pre-treatment (less white), as quantified in the middle graphic. Measures of function status show NK protection (as in the Wu study).
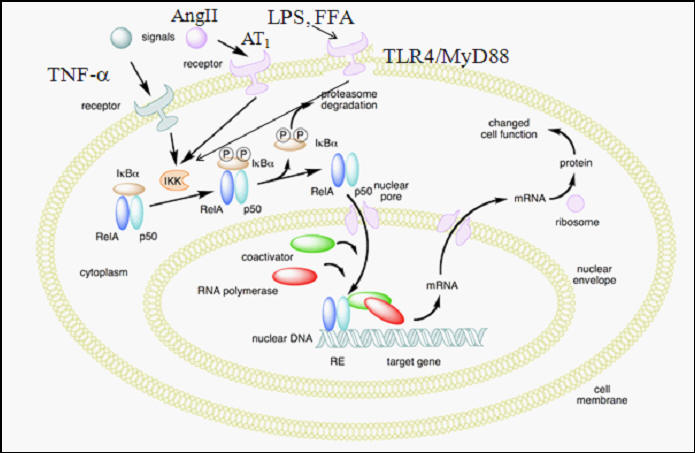
Legitimate infection (and inflammatory signaling in stroke that our body misinterprets as infection), activates the Nuclear Factor Kappa Beta (NF-kb) pathway, leading to the elaboration of a host of pro-inflammatory cytokines (Il-6, Il-1b, TNFa, and iNO, pro-inflammatory nitric oxide). Bacterial products such as LPS (Lipopolysaccharide) and other threat signaling molecules will bind to the TLR4 receptor on a white cell, or free radical species, cytokines, or Ang II will promote degradation of IkBa, which restrains NF-kb in the cytoplasm, prompting translocation of NF-kb into the nucleus, where it activates pro-inflammatory gene expression.
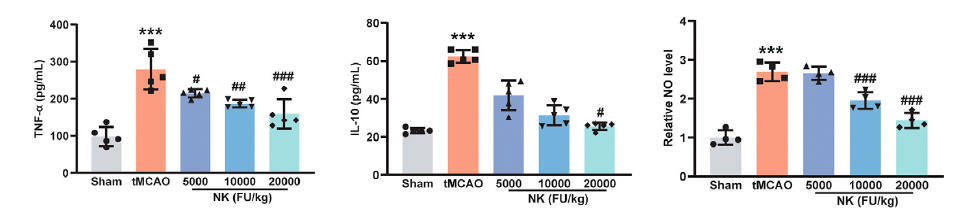
Dr. Yang found that NK blunts elaboration of the NF-kb coded inflammatory cytokines and iNO, in both brain tissue and in the serum.
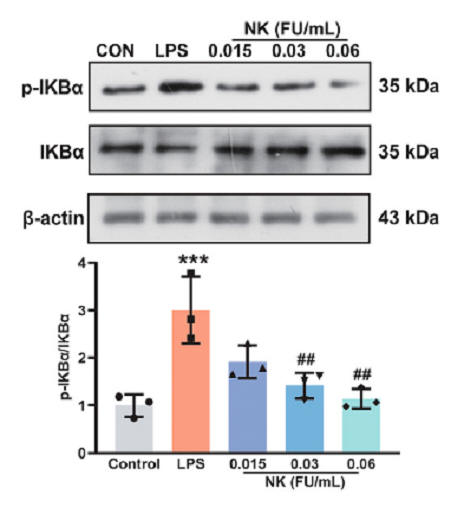
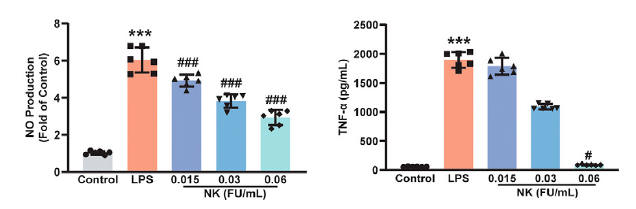
He then incubated white blood cells with LPS (which stimulates TLR4 and thus activates NF-kb) and found that NK protects against degradation of IkBa, measured as phosphorylated IkBa, blunting generation of downstream inflammatory mediators such as iNO and TNFa. Others studies tell us that NK actually degrades the TLR4, blunting inappropriate or smoldering inflammation. Monocytes within the nervous system are termed microglia, and just as monocytes in the periphery can adopt either a pro-inflammatory or anti-inflammatory status, so can microglia. NK shifts this bias towards an anti-inflammatory posture.
To help determine whether NK will attenuate neural inflammation and tissue damage in other settings, Yang exposed cells to OGD/R (oxygen and glucose deprivation followed by reperfusion), a standard cell stress methodology, and found that NK protects against cell loss (apoptosis). Cell suicide protein (Bax, Caspace, CHOP, and PARP) activation, due to stroke, OGD/R, and likley other causes of cell stress is attenuated by NK.
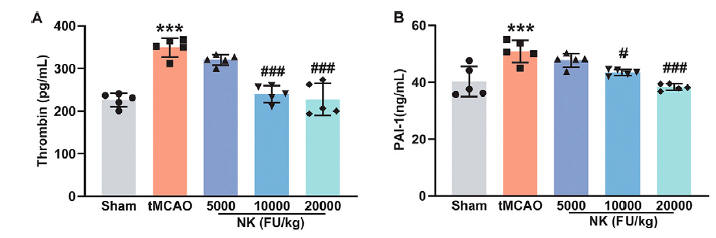
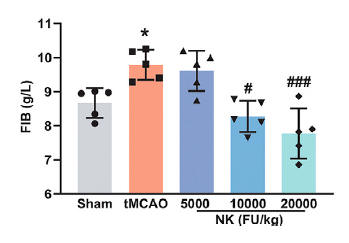
While stroke in this study was surgically induced, and not caused by a blood clot, inflammation and oxidative stress secondary to the stroke activated the pro-clotting proteins thrombin, PAI-1, and Fibrinogen, an effect that was attenuated with NK.
All of these stroke recovery, neurogenesis, anti-inflammatory and oxidative stress reduction effects of NK were canceled out with co-treatment when the NK was heat inactivated or if an agent that blunts proteolytic enzyme activity was co-administered.
Nattokinase supplementation most certainly will reduce one's risk of stroke (a
corresponding clinical trial will never be carried out, as NK is not
patentable), given its across-the-board risk factor reducing and
anti-atherosclerotic effects. If IV nattokinase were available, we could use it
interchangeably with rTPA. These studies tell us that NK improves outcome
after stroke by:
A. Generating Irisin, which increases BDNF, which leads to neurogenesis.
B. Blunting oxidative and inflammatory stress, shifting the microglia from pro-
to anti-inflammatory in function.
C. Protecting against apoptosis (cell suicide).
D. Blunting the link between inflammation/oxidative stress and enhanced
clotting.
These properties should protect all of our cells, within all of our organs, from
the stress of other health challenges.
Yang, X. et. al. Antioxid. Redox Signal. 42, 228-248.
Effect of Nattokinase in DVT Prevention and Chronic Venous Insufficiency
Given the anti-inflammatory, clot lysing, and endothelial support characteristics of Nattokinase (NK), we would expect NK to be of value in preventing DVT (Deep venous Thrombosis) and in CVI (Chronic Venous Insufficiency). Drs. Ceasareone and Gallelli put this hypothesis to the test in the studies abstracted below.
Within our venous circulation, we are constantly forming micro-clots, and then dissolving them (NK facilitates the latter action). DVT occurs when phenomena that promote clot formation overwhelm our intrinsic thrombolytic (clot lysing) defenses. DVT relates to an interaction between venous endothelial dysfunction (suboptimal local TPA elaboration), inflammation (which triggers coagulation), and venous stasis (sluggish lower extremity blood flow) due to prolonged inactivity (bed rest or travel). As such, if you are placed at best rest, we often treat you with subcut heparin or an oral anticoagulant. This action protects you from DVT, but also exposes you to drug-related bleeding risk. With car travel, we ask you to take breaks, and get up and walk around; and to periodically pace the isles when on a long flight.
♥ Cesareone and colleagues randomized
204, New York to London fliers (none on blood thinners) to receive:
·
Flite Tabs (300 mg blend of Pycnogenol and
Nattokinase) two hours prior to lift off and again at six hours.
·
Matching placebo.
Venous ultrasound studies were carried out
at the NY an London airports, along with measurement of calf circumference (an
index of edema).
All were encouraged to periodically get up and walk the aisle.
Pycnogenol is a trade name for Grape Seed or Pine Bark extract, a bioflavonoid,
with known vascular protective and antioxidant effects.
5-10% of us will experience sub-clinical DVT during travel, which our body will then break down as we get up and moving. Rarely, the clot will grow, obstruct blood flow, and then we experience clinical DVT, requiring anti-coagulant intervention.
Ultrasound study in the placebo group
demonstrated:
· 5.4%
DVT (5 of 92) and 2 fliers with SVT
(superficial venous thrombosis), and overall thrombotic event rate of 7.6%.
· No
DVT or SVT occurred in the treatment
group.
Edema level increased in 12% in the placebo group and decreased by 15% in the Flite Tab group
Edema reduction with Pycnogenol is expected, but bioflavonoid supplementation alone would not be expected to prevent DVT. NK was likely the key here.
Prevention of Venous Thrombosis in Long-Haul Flights with Flite Tabs. Cesareone, M. et. al. Angiology 54:531-539, 2003.
♥ Gallelli and colleagues followed the course of 153 patients under treatment with various levels of chronic venous insufficiency
· 50 patients with acute DVT wert treated with subcut Fondaparinux (a Heparin-like agent) over four weeks followed by Nattokinase 100 mg (2000 FU) daily over an additional 30 days. Subjective symptom score (VAS, with 10 reflecting severe and 0 reflecting no symptoms) decreased from 8 to 5 with Fondaparinux and then to < 1 following 30 days of NK. VAS with digital pressure (pain with manual compression over the calf) decreased from 9 to 5 with Fondaparinux and then to nil with NK (see chart below).
· 57 patients with SVT (superficial venous thrombosis or phlebitis) received subcut Lovenox (another Heparin-like agent) over four weeks followed by Nattokinase 100 mg (2000 FU) daily over 30 days. Subjective symptom score fell from 7 to 3 and with digital pressure from 8 to 4 with drug therapy, and then to essentially nil with NK.
· 46 patients underwent vein ligation and stripping followed by Nattokinase 100 mg (2000 FU) daily over 30 days. Symptom scores fell from 8-9 to 1 at 30 days.
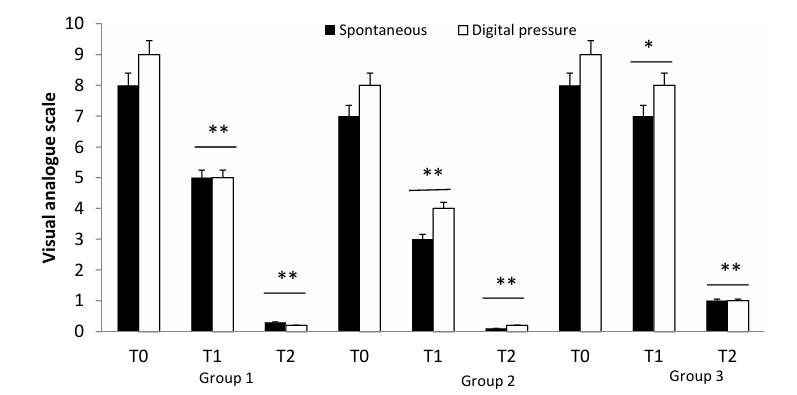
No side-effects or drug interactions were recorded; specifically abnormal bleeding did not occurred.
Data Recorded in Real Life Support the Safety of Nattokinase in Patients with Vascular Diseases. Gallelli, G. et. al. Nutrients 2012. 13, 2031.
Effect of Nattokinase on Spike Protein Clearance and Long Covid